Air is a gaseous mixture that circulates on the earth. Numerous hazardous toxic gases are suspended in the air along with various life-sustaining gases, including oxygen, which is necessary for humans, as well as nitrogen and hydrogen, which are crucial for plants to thrive.
These toxic gases include gases such as hydrogen chloride, benzene, dioxin, or compounds such as asbestos, as well as elements such as mercury, chromium, etc. Different gases in the environment can act as toxic gases if their concentrations exceed the allowable exposure limits. Even oxygen can do serious harm to the human body, known medically as oxygen toxicity. Oxygen exposure at partial pressures higher than the body’s typical exposure causes oxygen poisoning. Either severe or modest exposure levels to these hazardous gases can have negative effects on health. Let us know about these in detail.
Introduction

When you hear the term toxic gases, you may wonder what makes a gas toxic to humans. “Toxic gases are typically defined as having a median potentially deadly concentration exceeding 200 ppm,” according to GDS Corp, Houston, Texas. So, what exactly does median lethal concentration imply? A toxic unit that estimates the deadly dose of a toxin, radiation, or pathogen is the median lethal concentration. Gas detection experts say, “When toxic gases are absorbed, inhaled, or consumed through the eyes or skin, they can cause damage to living cells and tissues, damage to the nervous system, serious disease, or, in extreme cases, death.”
Different toxic gases can quickly accumulate in confined settings. For example, Some factory activities, such as soldering and welding, can also lead to toxic gas accumulation in a compact area.
Toxic Gases: their Types, Sources and Effects
Let us see different toxic gases present indoors and/or outdoors and their effects on an average human body:-
1. Nitrogen-
![]()
It is the most prevalent gas in the atmosphere. Nitrogen comprises more than 75% of the air we breathe. Therefore, N2 is the most lethal of all destructive, dangerous, and toxic gases. Since it is the gas that we breathe in the most, it is found abundantly in the atmosphere. Nitric oxide and nitrogen dioxide are substances that are present in many commercial and residential settings. Consequently, the primary causes of air pollution.
The most frequent sources of nitric oxides are industrial and vehicular emissions, fossil fuel combustion, agricultural operations, etc. whereas the manufacturing of explosives and rocket fuel uses nitrogen dioxide.
25 ppm (Parts Per Million) and 5 ppm is the current acceptable limit for nitric oxide and nitrogen dioxide for an 8-hour work shift respectfully. Whereas lethal concentrations are 100 ppm and 20 concentrations for nitric oxide and nitrogen dioxide.
Exposure symptoms include irritation in the eyes, skin, and respiratory system. When exposed for longer durations, in high concentrations they can lead to death as well.
2. Hydrogen Sulfide (H2S)
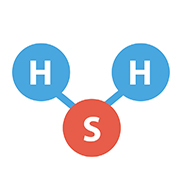
Sewage gas, often known as hydrogen sulfide (H2S), is a colorless, highly combustible gas that is also extremely hazardous. Even in small amounts, it has a distinctive “rotten egg” odor.
Mining, the production of pulp and paper, the production of rayon, the refinement of oil and gas, and other industrial processes all use H2S. In contrast, it occurs naturally in places such as natural gas wells, volcanoes, compost pits, and sewage. Other sources of Hydrogen Sulfide include natural gas processing and utilization, power plants, coal gasification, refineries, petrochemical processing, wastewater treatment, and semiconductor manufacturing. H2s can build up in confined areas like manholes, sewage systems, and telephone tunnels because it is denser than air. Working in small areas can be quite risky due to its presence. Furthermore, you lose the ability to smell it to alert you to its presence after a period making it more deadly.
Depending on how much and for how prolonged a laborer inhales H2S, different health impacts can result even in low quantities. Effects might range from minor eye irritation or headaches to very serious ones such as blackouts and fatalities.
Carbon Monoxide (CO)

Burning materials incorrectly releases co into the air. It is a tasteless, odorless, and colorless gas. Particularly in populated environments where human exposures cannot be regulated, CO is extremely toxic.
OSHA recommends an exposure limit of 50 ppm for 8 hours. Therefore, using cautionary methods if concentrations exceed 100 ppm becomes important. Lethal concentrations are above 200 ppm.
Health problems like nausea, restlessness, and euphoria can be experienced which might eventually result in death if the exposures are for a prolonged period.
Ozone (O3)
![]()
O3 is commonly regarded as smog in urban areas. High O3 concentrations at the ground levels are a result of oxidation reactions caused by volatile compounds and automobile emissions, quite apart from this occurring naturally in the upper atmosphere. It is exceedingly dangerous to both humans and plants.
0.10 ppm of exposure for eight hours in a day and these levels must never exceed this average as directed by OSHA. Although the intensity of the activity may influence the actual figure, use precautions while operating with O3 for an extended period.
It is a toxic gas that needs to be constantly regulated due to health issues like skin cancer, impaired breathing symptoms, sunburns, or chest illnesses in humans.
Solvents
Typical solvents include gasoline, kerosene, paint strippers, etc. Carbon-based organic solvents with high combustibility and toxicity can dissolve other chemical compounds.
High amounts can have a negative impact on your central nervous system. When exposed to solvents over an extended period, one may also experience other negative side effects such as tiredness, lack of attention, confusion, reproductive damage, liver and kidney damage, respiratory impairment, cancer, dermatitis, headaches, coma, and even death.
| – Some other chemically toxic gases used in industries that must be stored with proper ventilation according to the NFPA (National Fire Protection Association) toxicity ranking include: | |
|---|---|
| Toxic gases | Ranking |
| Arsine | 4 |
| Florine | 4 |
| Carbon Monoxide | 3 |
| Hydrogen Cyanide, Hydrogen Fluoride, Hydrogen Sulfide | 4 |
| Sulfur Dioxide | 3 |
| Ammonia | 3 |
| Ozone | 4 |
| Chlorine | 3 |
| Ethylene Oxide | 3 |
| Vinyl Chloride | 2 |
| Hydrogen Chloride, Hydrogen Bromide | 3 |
| Nitrogen Dioxide, Nitric Oxide | 3 |
What harm can toxic gases do?
An underperforming respiratory system is caused by these toxins, either individually or collectively. The condition increases the risk of respiratory tract infections such as bronchitis, and pneumonia. In the end, the sick people perform significantly worse and produce significantly less. To give humans a clean environment for respiration and a high productivity setting, therefore proper ventilation is required.
Strong oxidants which are very reactive substances, can immediately react with the epithelial lining of the airways and result in disorders such as necrosis. Whereas less reactive gases can cause capillary endothelial cell necrosis by diffusing through the epithelial without causing obvious damage (eg.- nickel carbonyl).

Smoke inhalation injuries are mostly caused by inadvertent gases such as CO and hydrogen cyanide (HCN) intake. CO also affects cellular level tissues, interfering with cell respiration. In addition, protein in both skeletal and cardiac muscle may bind to CO and result in severe toxicity.
Which toxic gases to avoid?
The answer is… All of them. When concentrations of the gases are above their permissible limits, they are considered toxic. Following is the list of toxic gases indoors and outdoors and their permissible limits.
| Toxic Gas | Found in | Found in | Guidelines set by |
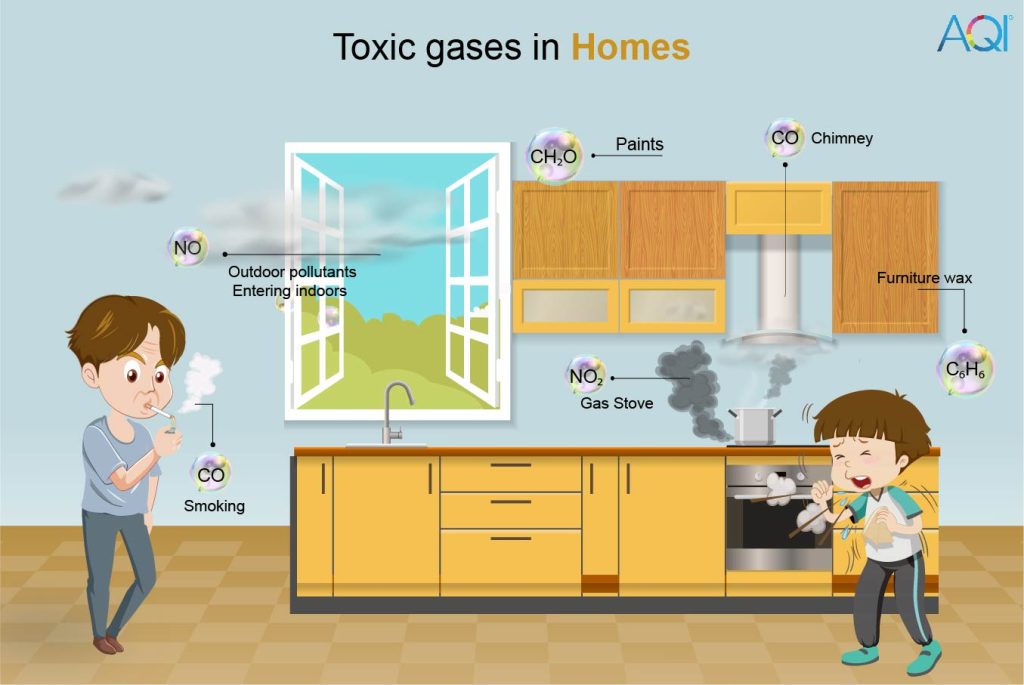
| Benzene | Indoor- glues, furniture wax, paint, and detergent. Outdoor- Industries | • There is no such thing as a safe level of exposure. • Component danger of acute leukemia is 6 x 10-6 per 1 g/m3 air concentration. | WHO |
| Carbon monoxide | Indoors- improper combustions of coal and gasoline Outdoors- Vehicular and industrial emissions | • 15 minutes – 100 mg/m3 • 1 hour – 35 mg/m3 • 8 hours – 10 mg/m3 • 24 hours – 7 mg/m3 | WHO |
| Formaldehyde | Indoors- building material, gas stoves, smoking, kerosene | 0.1 mg/m3 – 30-minute average | WHO |
| Hydrogen Sulfide | outdoors- coke oven plants, petrochemical plants Indoors- decomposition of human and animal waste | • 20 ppm (ceiling) • 50 ppm (peak- 8-hour shift) | OSHA |
| Nitrogen dioxide | Ambient- fuel burning, power plants Indoors- water heaters, fireplaces | • 200 μg/m3 – 1 hour average • 40 μg/m3 – annual average | WHO |
| Radon | Indoors- building materials Outdoors- water treatment facilities | • 0.6 x10-5 per Bq/m3 • 5 Bq/m3 to 15 Bq/m3 for outdoors | WHO |
| Arsenic | Outdoors- Industries, volcano eruptions, vegetation burning | 0.01 mg/m3 averaged over an 8-hour work shift. | OSHA |
| Hydrogen Cyanide | Indoors- blast furnaces, coke ovens Outdoors- Paper, rubber and synthetics burning | • 4.7 ppm (REL) • 4.7 ppm (TLV) | •NIOSH •ACGIH |
| Gasoline | Indoors- gas stoves Outdoor- Industries that require burning of gasoline | 8-hour TWA of 300 ppm | OSHA |
How do these affect wildlife and contribute to air pollution?
Toxic gases are dangerous to humans, wildlife, and vegetation. Some pollutants, such as lead and mercury, linger in the ecosystem for several decades and collect over time. Airborne toxins are contaminants that are known to cause disorders such as cancer, other major health issues, or environmental harm at sufficient amounts and exposure levels.

Even though everyone is at risk of being exposed to harmful gases, a variety of factors influence how badly a pollutant will damage an individual or community at risk. These include factors such as the intensity, length, and degree of exposure, the pollutant’s toxicity, and the exposed majority’s general health.
Certain toxic gases, for instance, mercury, can accumulate in topsoil or water bodies, where plants absorb them, animals and fishes eat them, and they move up in the food chain. Furthermore, animals can develop health issues if they are exposed to enough hazardous gases over time, just like people can.
Air pollution occurs when these toxic gases accumulate together to form hazardous concentrations. These concentrations are harmful to humans, wildlife, and the whole ecosystem. If the amount of a certain toxic gas increases in the atmosphere or a confined space, the air pollution levels of that respective area will also increase. Toxic gases are products or by-products of many processes in the ambient environment. For example, ground-level ozone is a by-product of a chemical reaction between vehicular emissions, heat, sunlight, and VOCs.
How to know if you are inhaling a toxic gas?
Consider the following pointers as an indicator that you might be inhaling toxic gases:
- If you are feeling sick for example headaches, for longer intervals
- Your home is near an industrial area
- If you have small factories
- You live near a construction site
- Gases such as Hydrogen Sulfide have a pungent smell. So, you will know when you inhale them.
- You have sudden symptoms of eye, nose, and throat irritation
- You experience abnormal health effects such as shortness of breath or blood in the sputum.
- Experiences of nausea and dizziness
Most toxic gases are colorless and odorless, which makes it difficult to know if you are inhaling a toxic gas or not. This makes keeping an eye on and monitoring these toxic gases easier, especially if you have any of the above-mentioned pointers. In that case, you would want to monitor the levels of these toxic gases.
Can they kill you?
The answer is yes, without a doubt. Severe exposures to toxic gases can result in damage to the body parts such as extremely bad, irreversible lung conditions and even death. Although there is some healing potential for the lungs, not all damage can be repaired.
They have an impact on a variety of organs and systems, according to experiments. Due to their chemical persistence and capacity to be consumed by fatty tissues, therefore retained in the body, dioxins have a lengthy half-life once they enter the body. Their life is estimated to be 7 to 11-year in the body.
Tips to avoid toxic gases exposure
We are exposed to toxic gases on a daily basis indoors as well as outdoors. Regardless of our numerous efforts, we inhale these toxic gases each day in one form or another. and we are unaware of their impact on our health. If you are exposed to any harmful or toxic gases, the following are some tips that you can follow to detoxify your body. They will not 100% detoxify your body of these harmful toxins, but they will surely help. These are:
- When you are at home-
- Reduce exposure to additional chemicals.
- Drink Lots Of Fluids and Perspire WHILE YOU’RE AT IT!
- Consume whole and nutrition-rich food products such as whole wheat, barley, etc.
- REMOVE Sweet and refined foods
- At power plants / factories / industries-

- Check for toxic gas contamination at industrial plants.
- Instruct workers about the dangers of poisonous/toxic gases.
- Machinery containing toxic gases should be stored in a different area.
- Establish a decontamination facility so workers can take a shower and remove their uniforms before exiting the work area.
- Make sure that no one was drinking inside a space where toxic gases such as H2S are being contained.
- Conduct health checks and fitness inspections for workers who have to use breathing masks.
Solutions-
Many indoor and outdoor sources can lead to toxic gas exposures, even when unaware. Some toxic gases have a pungent smell like hydrogen sulfide, while many others are colorless and odorless. That makes it extremely difficult to know if you are inhaling TOXIC GAS or not. Therefore, it becomes essential to monitor the concentration levels of such gases, so that necessary precautions can be taken. See overall AQI levels to know the quality of the air you are breathing.
Toxic gas monitoring is extremely necessary, especially when you are dealing with toxic substances daily. Let us say a factory, industry, or construction site where workers are exposed to toxic gases daily. Landfills also contribute to the toxic gas emissions in the atmosphere and the laborer working in landfills are exposed to these toxic gases daily. Prana air gas sensors can sense such toxic gases and they can be integrated into our monitors. For example, CO2, SO2, CO, H2S, and many more.
Alerting, detecting, and monitoring toxic gases in industries are very important. Workers must be well equipped with proper equipment such as gas masks and uniforms to prevent exposure to industrial emissions. Furthermore, the general public is also exposed to toxic gases both indoors and outdoors. Therefore, indoor and outdoor monitoring devices and proper masks are necessary to maintain the general fitness of individuals.
Conclusion
Toxic gases are deadly, it doesn’t matter how much concentration and for how long you are exposed to them. Exposures of toxic gases are not something that we experience in factories only, but we inhale toxic gases in our day to day. Gases such as nitrogen, carbon monoxide, hydrogen sulfide, etc. that are present in the ambient environment are harmful to our health as well.
Proper detection and monitoring methods should be used to know if you are exposed to certain toxic gases or not. Therefore, detection and monitoring become much more important if you are having symptoms of exposure. Additionally, if you are exposed, proper measures must be taken to prevent the harmful after-effects of gas exposure. If you work in an industry where you come across different toxic gases, use proper precautions such as gas masks.